This all-in-one online Grams to Atoms Calculator performs calculations using a formula that relates the mass of a substance in grams to the number of. This video explains how to calculate the number of moles of an element given the mass as well as how to calculate the mass given the number of moles.

Mole Ratios Mole Ratio Relationship
Calculate the number of moles and number of molecules of following.

. To get atoms from. 5988 kg 5988 g. Multiply the ratio obtained in Step 2 with the given number of moles of the.
1 mole of S atoms 6022 10 23 S atoms. 1 mole of pennies 6022 10 23 pennies. To Calculate Number of Molecules in a Given mole.
Dividing both sides by eqRT eq causes this to cancel from the right side. The number of moles of water produced is equal to the number of moles of hydrogen gas consumed. This lecture is about how to find the number of moles in chemistry.
Using a calculator divide the number of grams by the molar mass. In order to find the number of moles here we will have to divide the given number of particles by Avogadros constant. Mass of 0325 gram.
Then number of moles n 056. Use the ideal gas law to calculate the number of moles of the sample. Its easier to work with grams so convert the mass.
Ask me questions on Facebook. In this animated lecture I will teach you about the 3 different easy methods to calculat. Grams to Atoms Calculator.
First you must covert your grams to moles then you can take the moles and covert to atoms. Calculate the molar mass by multiplying the number of atoms of each element in the. Calculate the number of moles of carbon dioxide molecules in 22 g of CO 2.
Finding the number of moles Question. If you take your 878 grams of fluorine and then look at the atomic mass. 1 mole of Cu 2 S molecules 6022 10 23 Cu 2 S molecules.
The mass of one mole of is. Molecular mass of methane CH. Find the number of grams in 0700 moles of hydrogen peroxide H 2 O 2.
A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons. The result is the number of moles in your element or. How do you convert grams to moles step by step.
So n 34 x 10 23 6022 x 10 23. Find out the number of moles in 0325 grams of barium hydroxide. To give an idea of how.
0032 mg of methane. How to Calculate Moles. As you already know how the grams to moles conversion work find the number of moles.
Thus the known information is. N 5988 g. A r relative atomic mass of C 12 A r of O 16.

Molarity M The Concentration Of A Solution As The Number Of Moles Of Solute Chemistry Education Biology Notes Chemistry

How To Calculate The Number Of Moles The Formula For The Number Of Moles Is Mass Divided By Molar Mass Teaching Chemistry Chemical Science School Activities
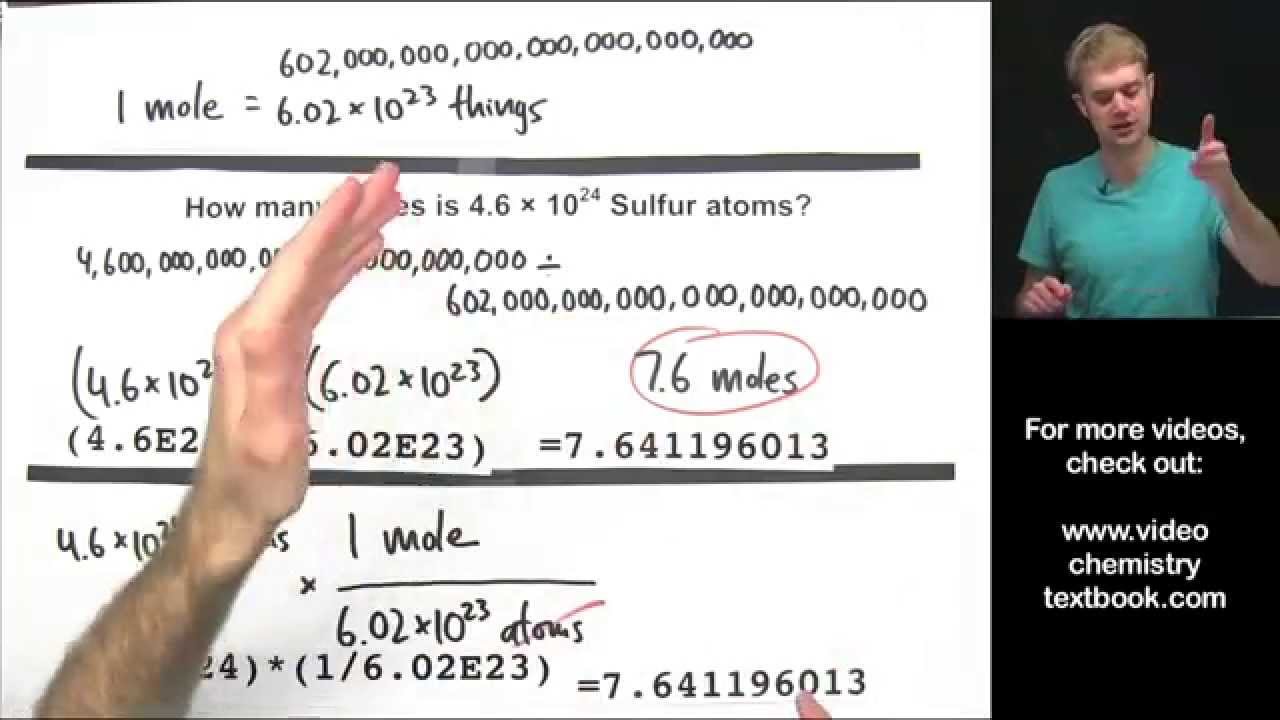
Converting Between Moles Atoms And Molecules Teaching Chemistry Chemistry Textbook High School Chemistry
